FDA Asks Malvern’s Endo to Pull Opioid Pain Med from Shelves
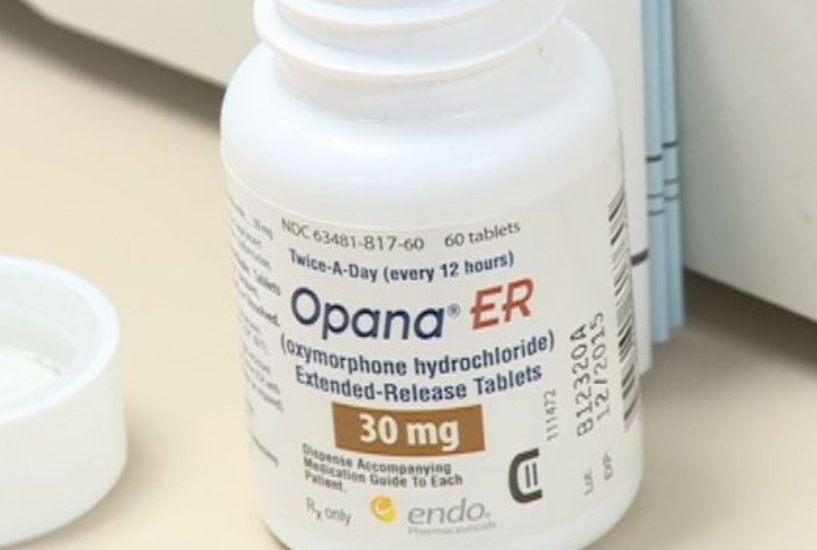
 The rampant drug abuse linked to a Malvern-based opioid pain medication has led to the U.S. Food and Drug Administration’s first-ever call to pull it from the shelves.
The rampant drug abuse linked to a Malvern-based opioid pain medication has led to the U.S. Food and Drug Administration’s first-ever call to pull it from the shelves.
The risks of abuse now outweigh the benefits of Opana ER, made by Endo Pharmaceuticals, according to a Philadelphia Business Journal report by John George.
“We are facing an opioid epidemic — a public health crisis — and we must take all necessary steps to reduce the scope of opioid misuse and abuse,” said FDA Commissioner Dr. Scott Gottlieb.
[uam_ad id=”58459″]
Advertisement
Injection abuse of reformulated Opana ER has been linked to an HIV, hepatitis C, and blood disorder outbreak.
“Despite the FDA’s request to withdraw Opana ER from the market, this request does not indicate uncertainty with the product’s safety or efficacy when taken as prescribed,” a company statement asserted.
“Endo remains confident in the body of evidence established through clinical research demonstrating that Opana ER has a favorable risk-benefit profile when used as intended in appropriate patients.”
Read more about the FDA’s request concerning Opana ER in the Philadelphia Business Journal here, and check out previous VISTA Today coverage of Endo here.
[uam_ad id=”58462″]
Connect With Your Community
Subscribe to stay informed!
"*" indicates required fields





















![95000-1023_ACJ_BannerAd[1]](https://vista.today/wp-content/uploads/2023/03/95000-1023_ACJ_BannerAd1.jpg)


























