West Chester Pharmaceutical Company Seeks Fourth FDA Approval for Skin Treatment
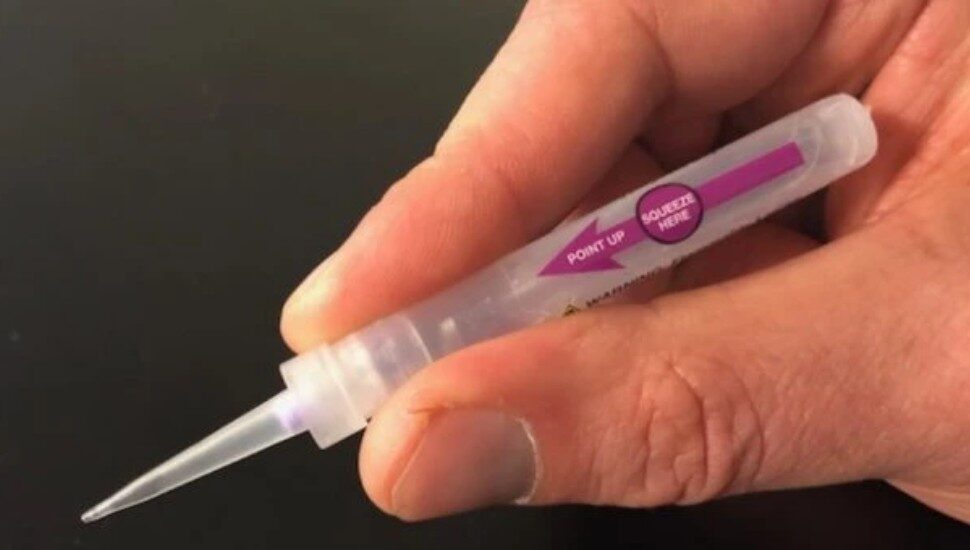
West Chester’s Verrica Pharmaceuticals is seeking FDA approval to market the drug “Ycanth,” which treats a skin condition, writes John George for the Philadelphia Business Journal.
Verrica is reapplying for the fourth time after its proposal was rejected last May due to manufacturing issues.
The condition, called molluscum contagiosum, results in contagious lesions on the skin. It’s caused by the pox virus and affects over 6 million people in the U.S. The medication is a drug-device product and delivers cantharidin through an applicator. It’s also in the process of being tested to treat common warts.
“We look forward to working with the FDA through the review process, and if approved, bringing VP-102 to patients as the first FDA-approved treatment option for molluscum,” said CEO Ted White.
Read more about the West Chester pharma company’s fight to seek FDA approval in the Philadelphia Business Journal.
Connect With Your Community
Subscribe to stay informed!
"*" indicates required fields












































![95000-1023_ACJ_BannerAd[1]](https://vista.today/wp-content/uploads/2023/03/95000-1023_ACJ_BannerAd1.jpg)



