Paoli-Based Startup Seeks FDA Approval for Device That Can Identify 34 Illnesses
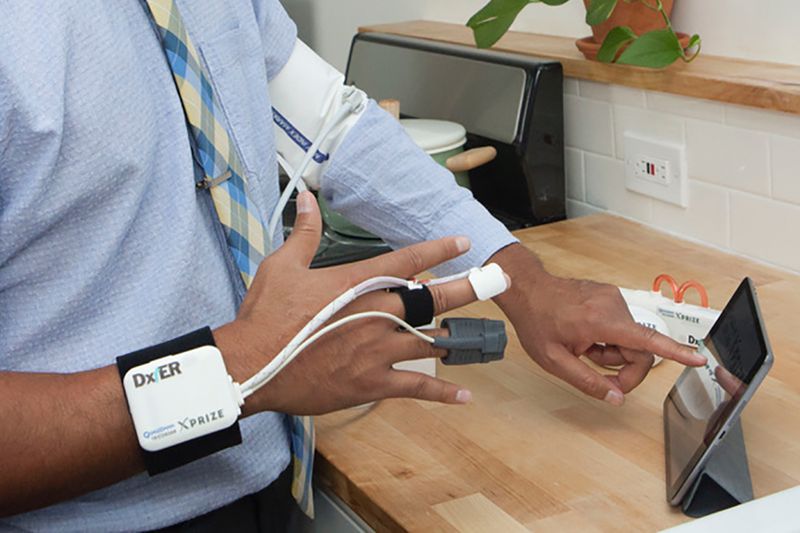
Basil Leaf Technologies of Paoli is seeking FDA approval to market its DxtER, a medical device that functions like the Tricorder from Star Trek in diagnosing illnesses, writes Michael Belfiore for Bloomberg Businessweek.
The shoebox-sized DxtER kit comes with a computer tablet equipped with an app and various Bluetooth-activated sensors, including a chest patch, finger probe, spirometer, and a combination thermometer-stethoscope.
Users launch the app on the tablet and, using an automated dialogue, apply the various sensors. The DxtER collects data and returns a diagnosis or indicates that it did not detect any illness.
[uam_ad id=”58459″]
Advertisement
The DxtER is able to identify 34 illnesses, including diabetes, atrial fibrillation, urinary tract infection, sleep apnea, and pneumonia.
Basil Harris, the co-founder and CEO of Basil Leaf Technologies, is an emergency room doctor and mechanical engineer. He formed Basil Leaf with his siblings and friends in 2012 to compete for the Qualcomm Tricorder XPrize, which it won last year.
Basil Leaf is working on DxtER upgrades that will enable it to diagnose as many as 75 conditions.
Read more about Basil Leaf Technologies in Bloomberg Businessweek by clicking here.
[uam_ad id=”72816″]
Connect With Your Community
Subscribe to stay informed!
"*" indicates required fields




![95000-1023_ACJ_BannerAd[1]](https://vista.today/wp-content/uploads/2023/03/95000-1023_ACJ_BannerAd1.jpg)