Malvern-Based Med Tech Company Good to Go for Wider Usage of Its OCD Treatment After FDA Clearance
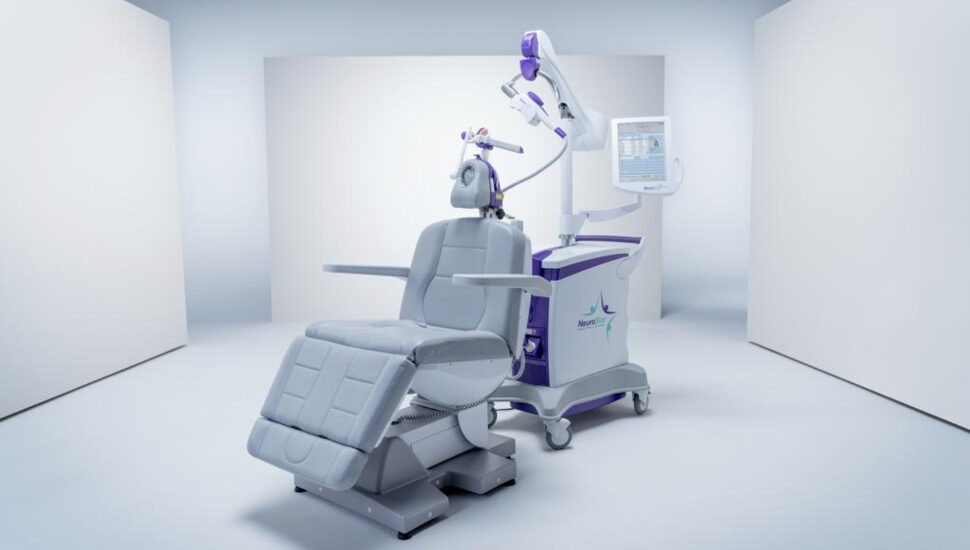
Malvern-based Neuronetics received clearance from the FDA for the wider use of its NeuroStar treatment system, writes John George for the Philadelphia Business Journal.
The medical technology company can now market the system, which delivers transcranial magnetic stimulation to the brain, as an adjunct therapy for adult patients who are suffering from obsessive-compulsive disorder.
NeuroStar is currently used as a major depressive disorder therapy. The system activates cortical and deep brain structures that are involved in mood regulation by using highly focused MRI-strength magnetic field pulses. The treatment can be delivered in under 19 minutes in a physician’s office.
“This new indication means that NeuroStar can help even more people suffering from mental health disorders that can be debilitating in their daily lives,” said Cory Anderson, vice president for research and development at Neuronetics.
The system’s design will make it possible for providers to treat this major depressive disorder and obsessive-compulsive disorder safely and without the need for any additional hardware upgrades or buying new equipment.
Obsessive-compulsive disorder afflicts about four million people in the U.S. The mental health disorder is marked by uncontrollable thoughts and fears that can lead to repetitive behavior or compulsions.
Read more about Neuronetics in the Philadelphia Business Journal.
Connect With Your Community
Subscribe to stay informed!
"*" indicates required fields






























![95000-1023_ACJ_BannerAd[1]](https://vista.today/wp-content/uploads/2023/03/95000-1023_ACJ_BannerAd1.jpg)

















