FDA Grant Boosts Exton-Based Castle Creek Biosciences’ Effort to Treat ‘Butterfly Children’
Exton-based Castle Creek Biosciences has received a $1.8 million grant from the Food and Drug Administration to support late-stage clinical testing of FCX-007. The experimental gene therapy is intended to relieve a rare skin disease afflicting children, writes John George for the Philadelphia Business Journal.
This is the second grant awarded by the FDA for this therapy. Castle Creek previously received a clinical trial research grant of $1.4 million.
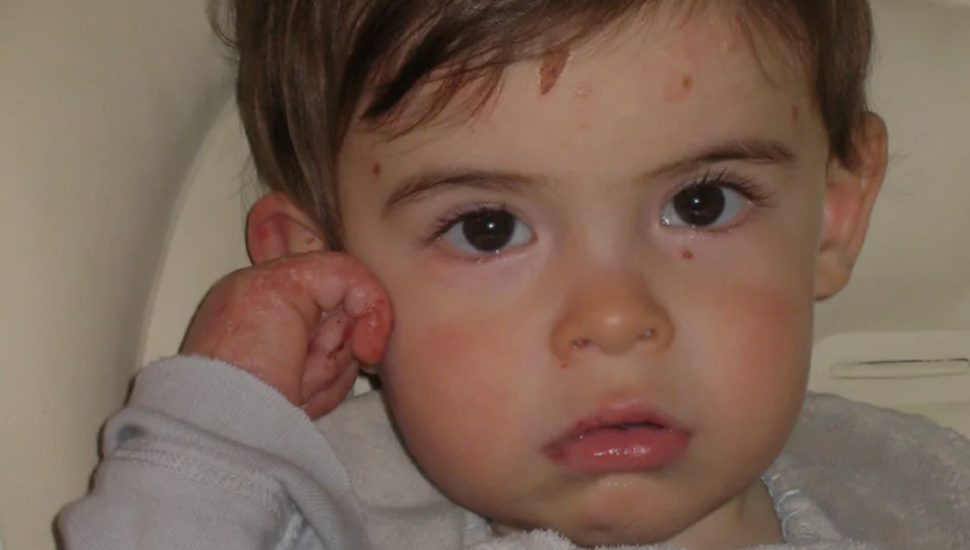
The company is developing experimental gene therapy as a potential recessive dystrophic epidermolysis bullosa (RDEB) treatment. The condition causes blistering, open wounds, and scarring in response to friction.
The disease, which impacts children from birth, has a high mortality rate. Due to their fragile skin, young patients with this disease are known as “butterfly children.”
According to Dr. Mary Spellman, Castle Creek CMO, the company’s treatment has the potential to completely transform the lives of people afflicted by RDEB.
“We are committed to advancing our study of FCX-007 to develop a durable personalized therapy for the localized treatment of chronic wounds due to RDEB and will continue to work closely with the FDA as our program progresses,” she said.
Read more about Castle Creek Biosciences in the Philadelphia Business Journal.
Connect With Your Community
Subscribe to stay informed!
"*" indicates required fields
























![95000-1023_ACJ_BannerAd[1]](https://vista.today/wp-content/uploads/2023/03/95000-1023_ACJ_BannerAd1.jpg)