FDA Decides Risks Outweigh Benefits of Endo’s Opioid Painkiller
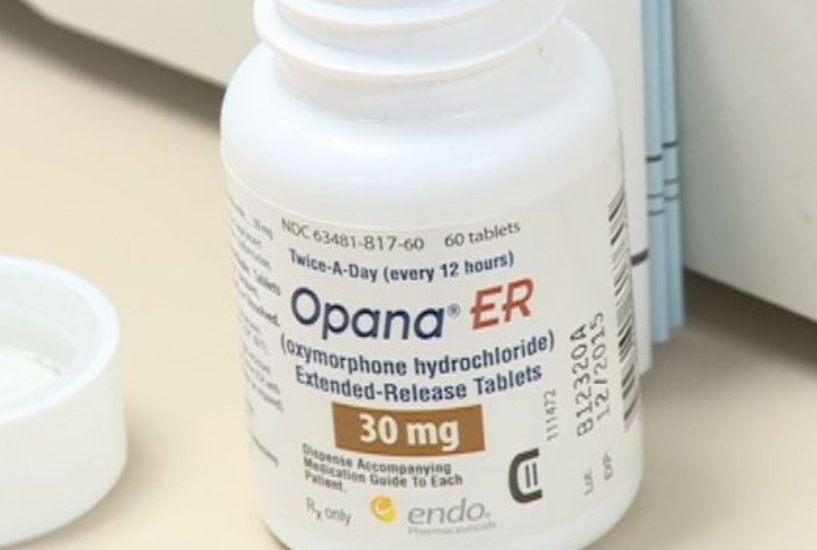
 A Food and Drug Administration panel has found that the benefits no longer outweigh the risks of Endo International’s opioid painkiller, Opana ER, writes John George for the Philadelphia Business Journal.
A Food and Drug Administration panel has found that the benefits no longer outweigh the risks of Endo International’s opioid painkiller, Opana ER, writes John George for the Philadelphia Business Journal.
The Drug Safety Risk Management and Anesthetic and Analgesic Drug Products Advisory committees voted 18-8 against the drug and its potential for abuse. However, Endo, which has its U.S. headquarters in Malvern, is still waiting for the FDA to make a final decision on any possible regulatory actions against opioid painkillers.
“Endo remains confident that the body of evidence established through clinical research demonstrates that Opana ER has a favorable risk-benefit profile,” said Dr. Matthew W. Davis, senior vice president for research and development for branded pharmaceuticals at Endo.
He added that the company’s top priorities include ensuring that patients with chronic pain have access to effective and safe therapeutic options.
“We plan to work collaboratively with the FDA as the agency completes its evaluation of Opana ER,” said Davis.
Opana ER was first approved 11 years ago, with the new formulation of the drug that is specifically designed to deter abuse introduced in 2012.
Read more about the FDA’s findings in the Philadelphia Business Journal here, and check out previous VISTA Today coverage of Endo International here.
Connect With Your Community
Subscribe to stay informed!
"*" indicates required fields






















![95000-1023_ACJ_BannerAd[1]](https://vista.today/wp-content/uploads/2023/03/95000-1023_ACJ_BannerAd1.jpg)

























