FDA Clears Way for 19-Minute Depression Treatment from Malvern’s NeuroStar
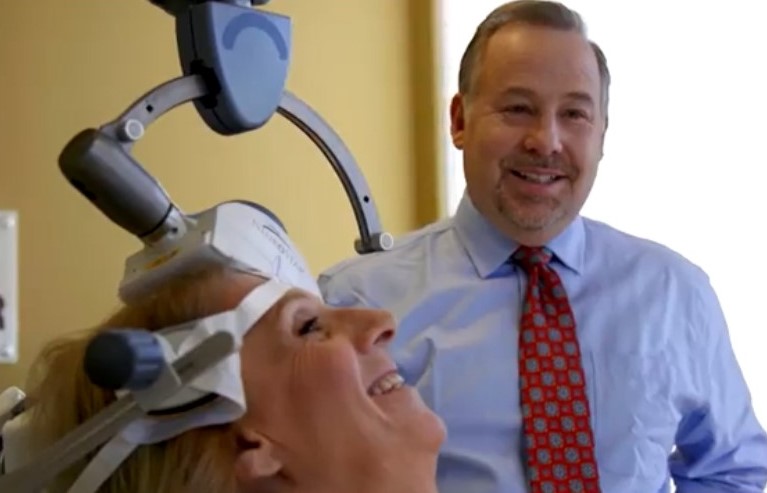
 Using up just a 19-minute block of time, it’s the fastest depression treatment of its kind, and now the U.S. Food and Drug Administration has cleared away the insurance hurdles for patients to get the NeuroStar Advanced Therapy.
Using up just a 19-minute block of time, it’s the fastest depression treatment of its kind, and now the U.S. Food and Drug Administration has cleared away the insurance hurdles for patients to get the NeuroStar Advanced Therapy.
“NeuroStar Advanced Therapy is a proven breakthrough treatment that has helped more than 50,000 people find relief from their depression, but many people still have not heard of it,” said President and CEO Chris Thatcher in a MassDevice report by Fink Densford.
[uam_ad id=”58459″]
Advertisment
“That’s because up until now patients struggled with insurance reimbursement and treatment scheduling challenges. Today, the most significant barriers to NeuroStar treatment are now gone.”
The FDA approval was a huge win for Malvern-based NeuroStar, which has had its advanced therapy transcranial magnetic stimulation device for major depressive disorder on the market since 2008.
“As a practicing psychiatrist, I see firsthand the incredible struggle that my patients have while trying to find the right treatment for MDD,” said Dr. Kenneth Pages of TMS of South Tampa. “NeuroStar Advanced Therapy is truly a breakthrough, non-drug treatment for MDD that changes the game for those patients who have seen failures with prescription medicine.
“I have confidence and comfort as I treat my patients because I know it allows for the right treatment dose to be delivered to the right location every time, giving my patients the best possible chance for long-term remission.”
Read more about NeuroStar’s milestone approval on MassDevice here.
[uam_ad id=”59343″]
Advertisment
Connect With Your Community
Subscribe to stay informed!
"*" indicates required fields









































![95000-1023_ACJ_BannerAd[1]](https://vista.today/wp-content/uploads/2023/03/95000-1023_ACJ_BannerAd1.jpg)